Dr Navin Ramachandran
Consultant Radiologist
Specialist expertise: Uro Ultrasound, Body CT, Body MRI, CT Urogram, Prostate MRI, Interventional Radiology, Radiology.
Our Imaging & Diagnostics centre is a beacon of excellence, offering an array of investigations to support our clinicians in accurately and quickly diagnosing patients’ conditions.
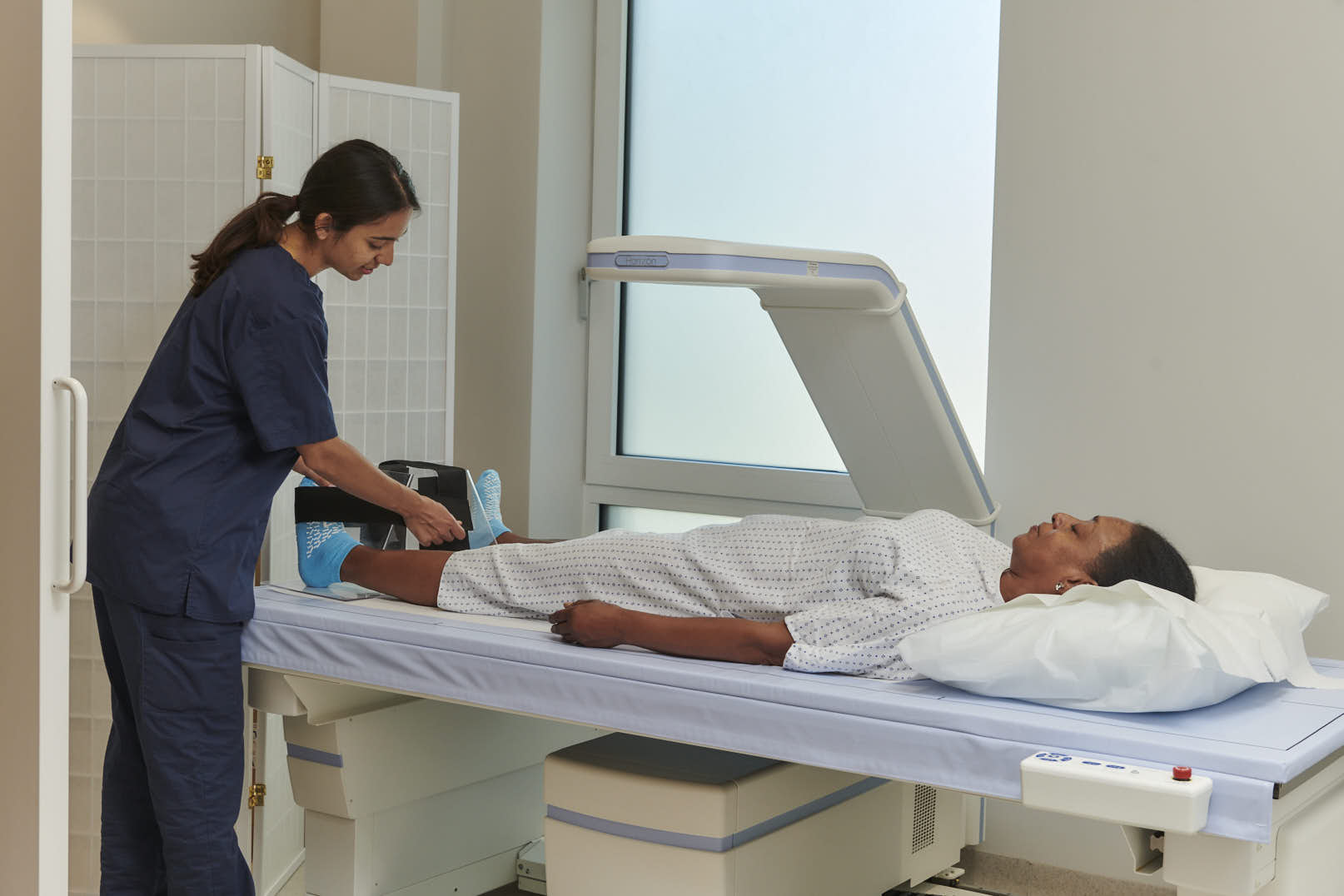
At OneWelbeck we have a state-of-the-art DEXA scanner, which is a vital piece of diagnostic kit used to assess bone density and a patient’s risk from osteoporosis.
Find out moreAll our consultant radiologists are highly trained in their specialist fields, which include musculoskeletal, cardiothoracic, gastrointestinal and breast. They have vast experience in designing and setting up imaging services at major teaching hospitals, and are leading authors in their respective fields of research.